Kyung Yup Baek
Korea Advanced Institute of Science and Technology, Korea
Title: A smart strategy for finding global minima of microhydrated biomolecules with minimal DFT calculations
Biography
Biography: Kyung Yup Baek
Abstract
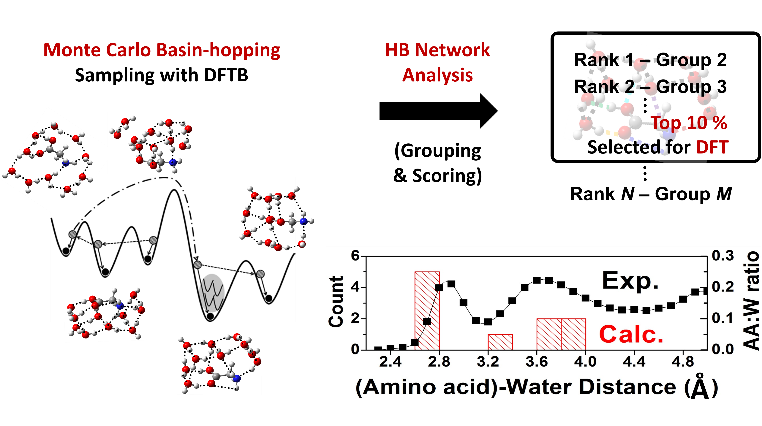
Water and biomolecules such as amino acids are the main building block of living organisms. Therefore, all phenomena and processes associated with them depend on the chemical interactions between these molecules in vivo. For example, it has been reported that tyrosine has a biological importance as a precursor of neurotransmitter called dopamine related to Parkinson's disease. Understanding their physicochemical behaviors in vivo is very challenging in terms of utilizing it for a new drug development. For this reason, biomolecule-water clusters as a model system have attracted significant attention in the scientific community. Undeniably, it is essential to identify stable structures and their patterns. However, there is no general way to elucidate stable hydrated structures even for simple amino acids because of the high complexity of chemical space increasing rapidly with the number of water molecules. In case of relying on chemical intuition only, it often failed to identify some important local minima, which could lead to misinterpretations about reaction dynamics of biomolecules in vivo. Here, we propose a very efficient computational method to selectively sample the most stable structures of the microhydrated biomolecules. The key idea is to utilize the unique structural patterns of H-bond networks obtained from their energetic features, i.e. their tendency to form more H-bonds. As a proof of concept, we could identify the new global minima of glycine•10(H2O) and for the first time, we found the minimum number of water molecules required to stabilize the zwitterionic form of tyrosine. Furthermore, the most stable structures of hydrated glycine and tyrosine indeed had common features, which were consistent with the X-ray data of proteins in water. Given the efficiency based on required DFT calculation amounts and accuracy, it is believed that our method give fast and accurate results for even more complex hydration systems.