Yeshayahu Ben-Eliyahu
Ben-Gurion University of the Negev, Israel
Title: Computational and empiric considerations regarding the electrocatalytic reduction of CO2 by water soluble cobalt porphyrins
Biography
Biography: Yeshayahu Ben-Eliyahu
Abstract
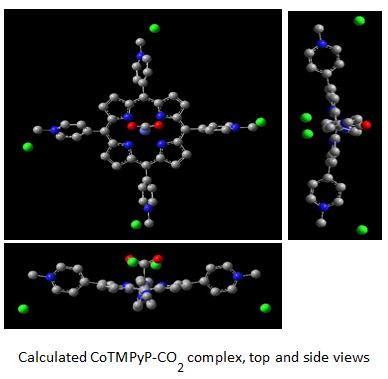
The electrochemical reduction of CO2 offers one of the possible solutions to current energy and sustainability issues since it can sequester carbon from the atmosphere and can be used to produce fuels and useful chemicals. In this respect, some metalloporphyrins have been reported to catalyze the electroreduction of CO2. However, key issues still remain in regard to the elucidation of the effect of the porphyrin structure on the reaction mechanism and catalyst activity. An essential and necessary stage in the proposed mechanism for the catalytic reduction of CO2 by the Co(II)/Co(I) porphyrin redox couple is the formation of an intermediate Co(II)porphyrin-CO2- complex. In an attempt to examine the effect of positively and negatively charged porphyrin substituents on the catalytic activity, we report here on a combined DFT and empirical study regarding the electrochemical reduction of CO2 in the presence of the Cobalt(II) 5,10,15,20-(tetra-N-methyl-4-pyridyl) porphyrin - Co(II) TMPyP and Cobalt(II) 5,10,15,20-(tetra-4-sulfonatophenyl) porphyrin – Co(II)TPPS complexes, with charges of +4 and -4, respectively. The lower catalytic activity of the CoTPPS complex as compared to that of CoTMPyP, both dissolved in aqueous alkaline solutions, as demonstrated by cyclic voltammetry experiments, are in agreement with the DFT study. Coulombic interactions seem to dictate the cobalt-carbon bond length and strength in the porphyrin-CO2 intermediate, and consequently have an impact on its stability and on the overall catalytic activity towards CO2 reduction.