Juan Manuel Lázaro-MartÃnez
University of Buenos Aires, Argentina
Title: Coordination chemistry in pyridine and imidazole compounds containing gem-diol moieties. Solid-state NMR and X-Ray studies
Biography
Biography: Juan Manuel Lázaro-MartÃnez
Abstract
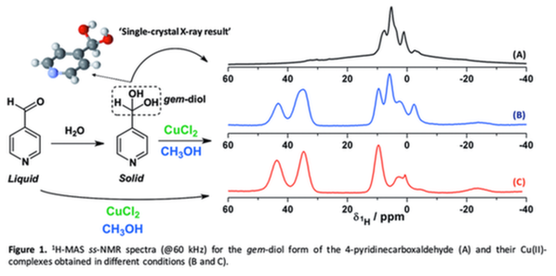
The need for clean technology either for fine chemistry or for waste treatment leads to the replacement of traditional inorganic oxidants such as K2Cr2O7 and KMnO4 by benign, easy-to-handle oxidants, such as H2O2 and O2. The activation of H2O2 can be achieved by transition-metal ion complexes with organic ligands. Particularly, H2O2 can produce OH• via the Cu(II)/Cu(I) cycle involving different reaction pathways. A high number of metal complexes bearing gem-diols has been reported, in which the presence of these moieties is generally demonstrated by single-crystal X-ray diffraction. As a rule, the stability of this functional group is not studied in the free ligand before the preparation of the metal complex. Understanding the chemistry of gem-diols is crucial for the development of synthetic methods to obtain new organic ligands, which are often used for the design of metal complexes with catalytic activity. In this context, solid-state NMR (ss-NMR) is a useful methodology to elucidate the chemical composition of mixtures in which both gem-diol and carbonyl forms are present in cases where the single-crystal cannot be obtained for X-ray studies. Additionally, the 1H-MAS ss-NMR spectra (@60 kHz) can also bring structural information about the ligands surrounding the paramagnetic center. To have an insight into the chemistry of gem-diol compounds, the aim of this work is to study the gem-diol generation and copper coordination properties in imidazole- and pyridinecarboxaldehydes through the combination of ss-NMR and single-crystal X-ray diffraction techniques. Complementary analyses were performed by solution-state NMR, high-resolution mass spectrometry (HRMS), and 1H ss- NMR. These studies allow us to expand the chemistry in metal complexes in terms of structural diversity of the ligands at the same time that new Cu(II)-homogenous catalyst towards the activation of H2O2 will be explored.