
Isa Degirmenci
Ondokuz Mayis University, Turkey
Title: Quantum chemical study on the tuning thiol-ene polymerization
Biography
Biography: Isa Degirmenci
Abstract
Recently, there has been a growing interest in the thiol-ene polymerization in many application areas from material science to bioorganic chemistry duo to the production of uniform polymer network, reduction of polymer shrinkage stress and obtaining narrow the Tg range. The most prominent feature of this polymerization is combination of the advantages of both step growth and chain growth polymerizations. The thiol-ene polymerization is involved in the initiation, propagation and chain transfer steps. This procedure is mostly governed by ratio of the propagation (kP) and chain transfer (kCT) reaction rates (kP/kCT). Recent experimental and theoretical studies have focused on ene functionality to evaluate the reaction procedure while the thiol functionality has been ruled out. However, this study has suggested that the thiol functionality also have to be taken into account to adjusting properties of a polymer. For this aspect, phenyl thiol derivatives have been considered for the thiol-ene polymerization of various monomers from electron deficient to electron rich alkenes. To evaluate kinetic and energetic features of the thiol-ene procedure, M06-2X/6- 31++G(d,p) level of theory was used for all geometry optimizations and frequency calculations. It was revealed that electrophilic nature of the phenylthio radicals and the stability of the RS-ene+ configuration predominates effect of the S-T gap of the ene functionality during addition reactions. Moreover, intermolecular interactions, such as π- π, have crucial role on the transition state of the chain transfer step. These interactions can diminish the strong influence of stability of intermediate carbon-centred radical on the chain transfer activation barrier. As a consequence, it was proved that the kP/kCT ratio is affected not only by ene functionality but also by the thiol functionality. This information can be taken into consideration for tailoring mechanical and physical properties of a polymer without changing the alkene structure to obtain industrially desirable polymer.
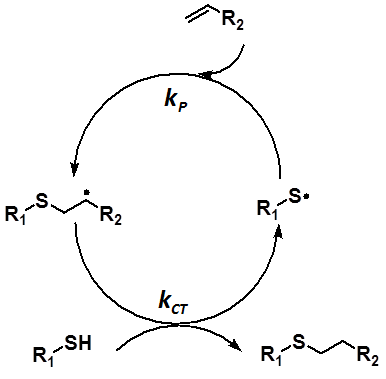
Scheme 1: Representation of the most significant steps of the thiol-ene reaction mechanism.
