Małgorzata A. Broda
University of Opole, Poland
Title: Structure and spectroscopy of E and Z isomers of Boc-Gly-ΔPhe-NHMe
Biography
Biography: Małgorzata A. Broda
Abstract
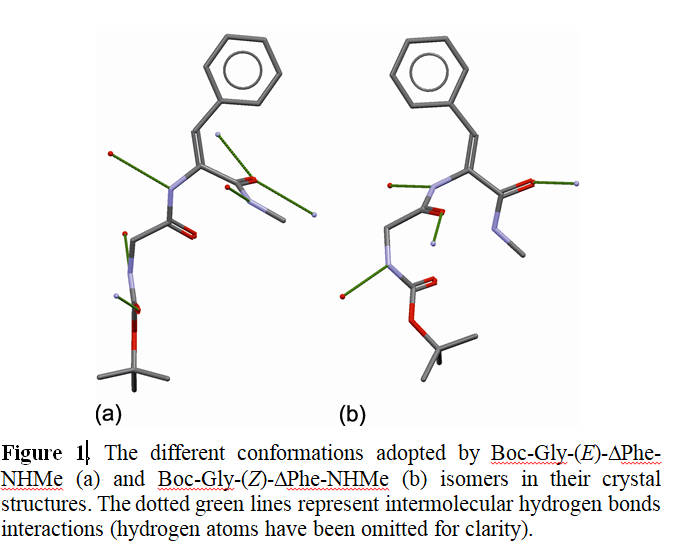
Biological activity of numerous small size molecules is directly related to their conformational properties. It is possible to control pharmaco-kinetic properties of naturally occurring peptides by introduction of nonstandard amino acid residues into their backbone chain which can result in analogues with improved pharmacological properties, such as resistance to enzymatic degradation, receptor selectivity, enhanced potency and bioavailability. For example, it is possible to introduce a dehydroamino acid residue and forcing a specific conformation of the chain fragment. Conformational properties of N-t-butoxycarbonylglycine-( E/Z)-dehydrophenylalanine N’-methylamides (Boc-Gly-(E/Z)- ΔPhe-NHMe) in chloroform were studied by NMR and IR spectroscopy. The low temperature crystal structure of the E isomer was determined by single crystal X-ray diffraction. The experimental findings were supported by extensive calculations at DFT (B3LYP, M06-2X) and MP2 levels of theory and the β-turn tendency for both isomers of the studied dipeptide were determined in vacuum and in solution. The obtained results reveal that the configuration of ΔPhe residue significantly affects the conformational properties of studied dehydropeptides. Theoretical conformational analysis reveals that the tendency to adopt β-turn conformations is much weaker for the E isomer (Boc-Gly-(E)- ΔPhe-NHMe), both in vacuum and in polar environment. We also showed a very good agreement between theoretical chemical shifts and calculated ones by using newly introduced relatively small and computationally inexpensive STO-3Gmag basis sets. The obtained results suggest a possibility of controlling dipeptide conformation by a simple chemical modification and thus allowing a future rational design of peptidomimetics.