Gregoire Guillon
University of Burgundy - Franche-Comté, France
Title: The O + O2 exchange reaction: symmetry, isotope effects, and influence of molecular forces
Biography
Biography: Gregoire Guillon
Abstract
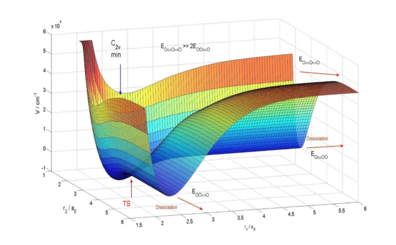
Statement of the Problem: Molecular oxygen O2 is the most important molecule in Earth’s atmosphere and stratospheric ozone O3 protects us from 97% of UV radiations. The abundance in 16O being 99.8%, O2 and O3 exclusively formed from it are dominant, thereby giving a reference for any process involving oxygen. A strong enrichment (about 10%) of O3 in both 18O and 17O (the socalled mass-independent fractionation MIF), has first been observed decades ago. The three body recombination O + O2 + M → O3 + M is believed to be the main process leading to this enrichment and at low pressures, it can be partitioned into two steps: the formation of O3 in a highly excited rovibrational state, from reaction O + O2 → O3*, and its subsequent stabilization by collision with an energy absorbing partner M (say N2 or O2), O3* + M → O3 + M. Thus, the efficiency of the exchange reaction O + O2 → O3* → O2 + O, involving metastable O3* as an intermediate, is one of the key parameters to understand ozone formation. This reaction is very fast and competes with the stabilization process.
Methodology: Using a newly developed, very accurate, potential energy surface (PES), we have realized computationally intensive full-quantum investigation of the dynamics of this process, using a time-independent formalism.
Results: We have, from first principles, computed reactive cross sections and reproduced measured rate constant for the 18O + 32O2 process, within experimental error bars. We will sum up resulting cross sections and rate constants for the various 16O + 32O2, 18O + 32O2, 17O + 32O2, 16O + 36O2 and 16O + 34O2 processes, discussing isotope effects and inclusion of permutation symmetry. We will discuss the strong influence of the PES.