
Concha Tojo
University of Vigo, Spain
Title: Slowing-down of Pt reduction kinetics in microemulsions: a computer simulation study
Biography
Biography: Concha Tojo
Abstract
The interest in developing new nanocatalysts composed by two metal components have been paid special attention, because the presence of a second metal gives rise to an improved catalytic behavior. In this line, Pt/M catalysts show high catalytic activity, which depends on the nanoparticle size, composition and metals segregation in the nanoparticle. A versatile method to control the size and composition of bimetallic nanoparticles is the micro emulsion route. But even here, the high number of variables make it difficult to tune the bimetallic nanostructure. As the ability to manipulate the metal distribution in bimetallic nanoparticles requires a deeper understanding of the mechanisms governing nanoparticle formation in microemulsions, we developed a simulation model to predict the atomic arrangement of bimetallic nanoparticles synthesized in microemulsions. The model was proved by comparing simulated nanostructures with Au/Pt nanoparticles. Excellent agreement between experimental and theoretical STEM profiles shows the validity of the model. On this basis, the model can be used not only as a tool to predict nanoparticles arrangement, but also as a means to further our knowledge of the kinetics in microemulsions. The purpose of this study is to perform a comprehensive kinetic analysis of the Pt/M nanostructures in the light of the interplay between three kinetic parameters: chemical reduction rates of the two metals, reactants concentration and inter micellar exchange rate. The combination of these factors determines the reaction rate of each metal, which in turn determines the final metal arrangement. We present results of Au/Pt (Pt: slower reduction) and Pt/Cu (Pt: faster reduction). Results show that Pt reduction rate not only depends on the usual parameters (Pt reduction rate, concentration, micro emulsion composition) but also on the reduction rate of another metal of the couple Pt/M. As a result, when Pt is the faster metal, final nanoparticle show a better metal segregation.
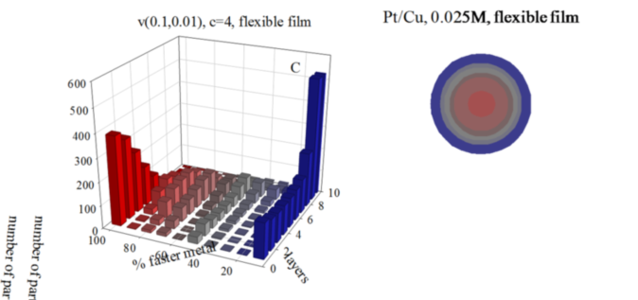
Figure: Histograms represent the number of particles with a given percentage of the faster metal in each layer, from the core to the surface, for different initial concentrations, and using a flexible film. Chemical rates: Au reduction is represented as an instantaneous reaction (100% of Au metal salts inside colliding micelles react); in Pt reduction only a 10% of Pt salts react in each collision; Cu, a 1% of Cu salts react. Scheme color: blue (0% - 45% of faster metal), grey (45%-55%), red (55% - 100%). Less red means less faster metal. Circles in each histogram represent nanoparticle structure in concentric layers, keeping the same color scheme
